A team of scientists created a novel type of temporary pacemaker — one that dissolves on its own, without requiring any removal. In their latest research, they’ve paired the pacemaker with a series of wireless sensors on the skin, which should allow it to smartly monitor a patient’s vital signs and adjust its pacing autonomously. Should the device continue to show promise, it could one day be used in patients undergoing cardiac surgery or who otherwise only need a pacemaker for a short while.
Last year, researchers at Northwestern University and George Washington University debuted the first version of the pacemaker.
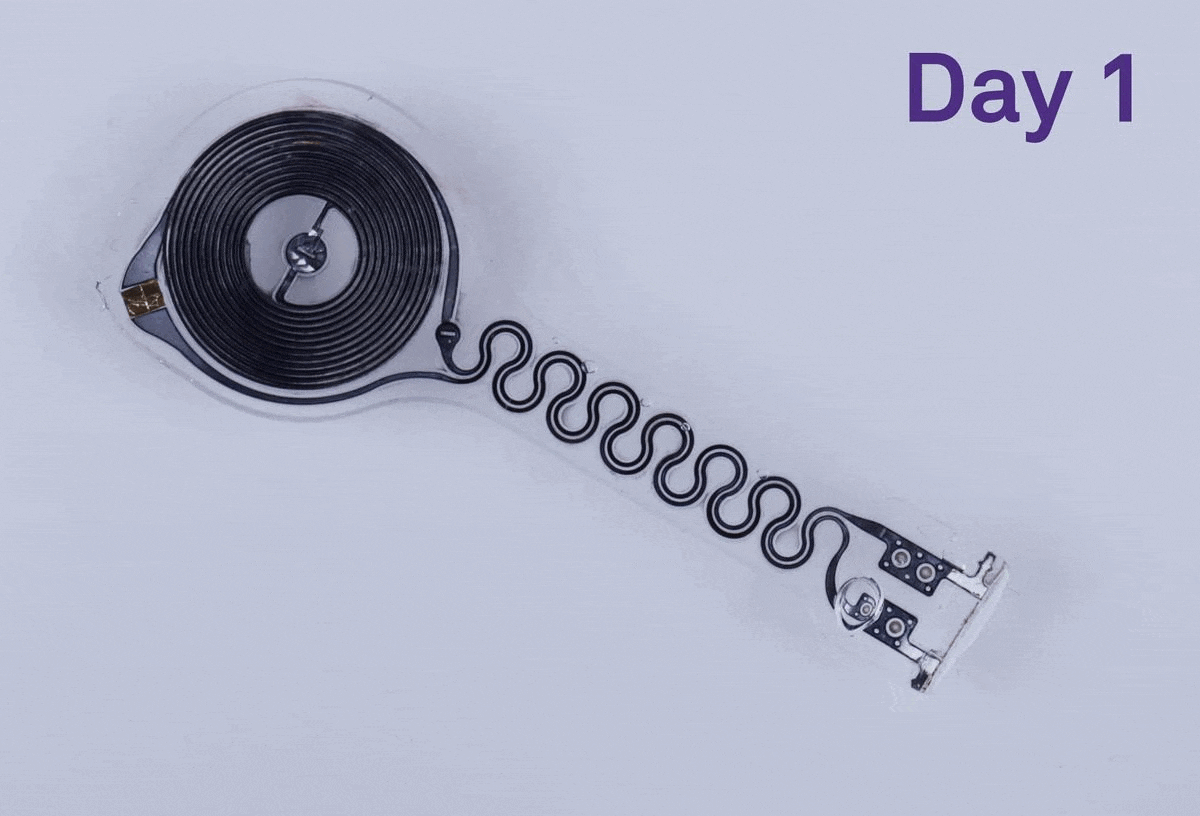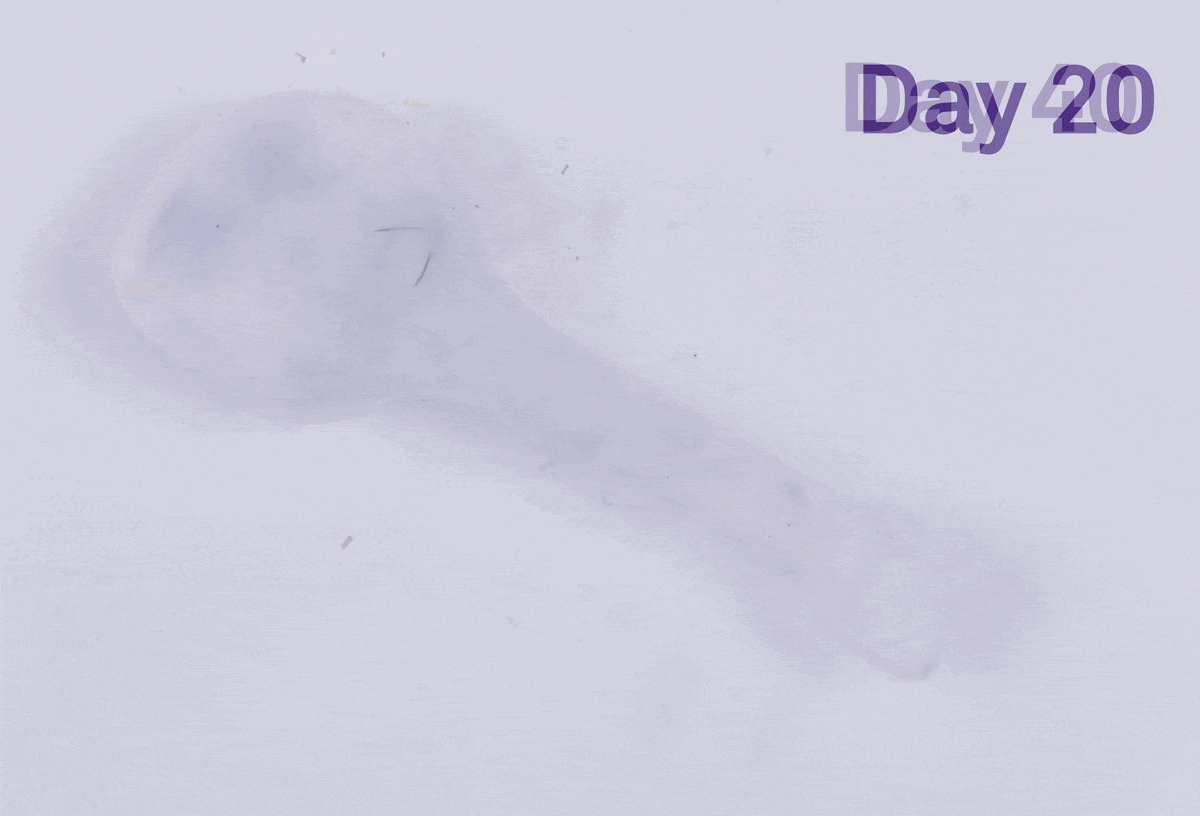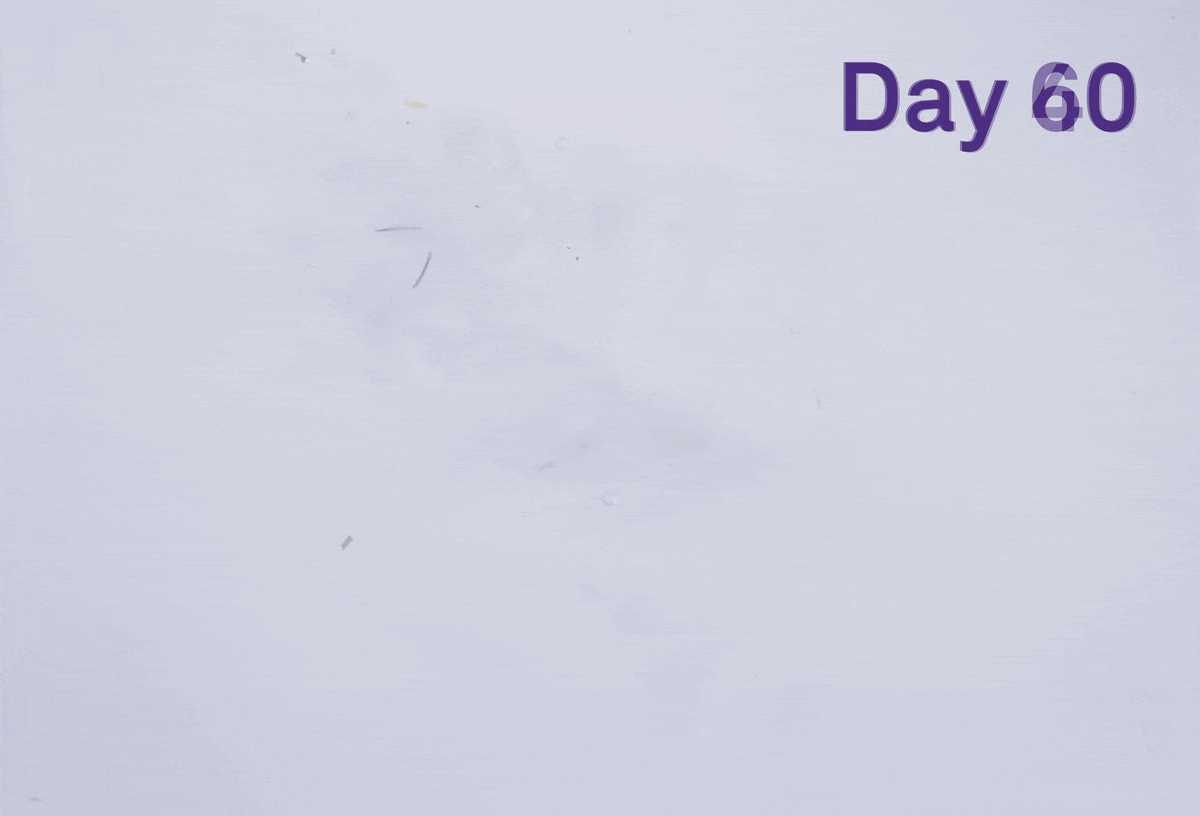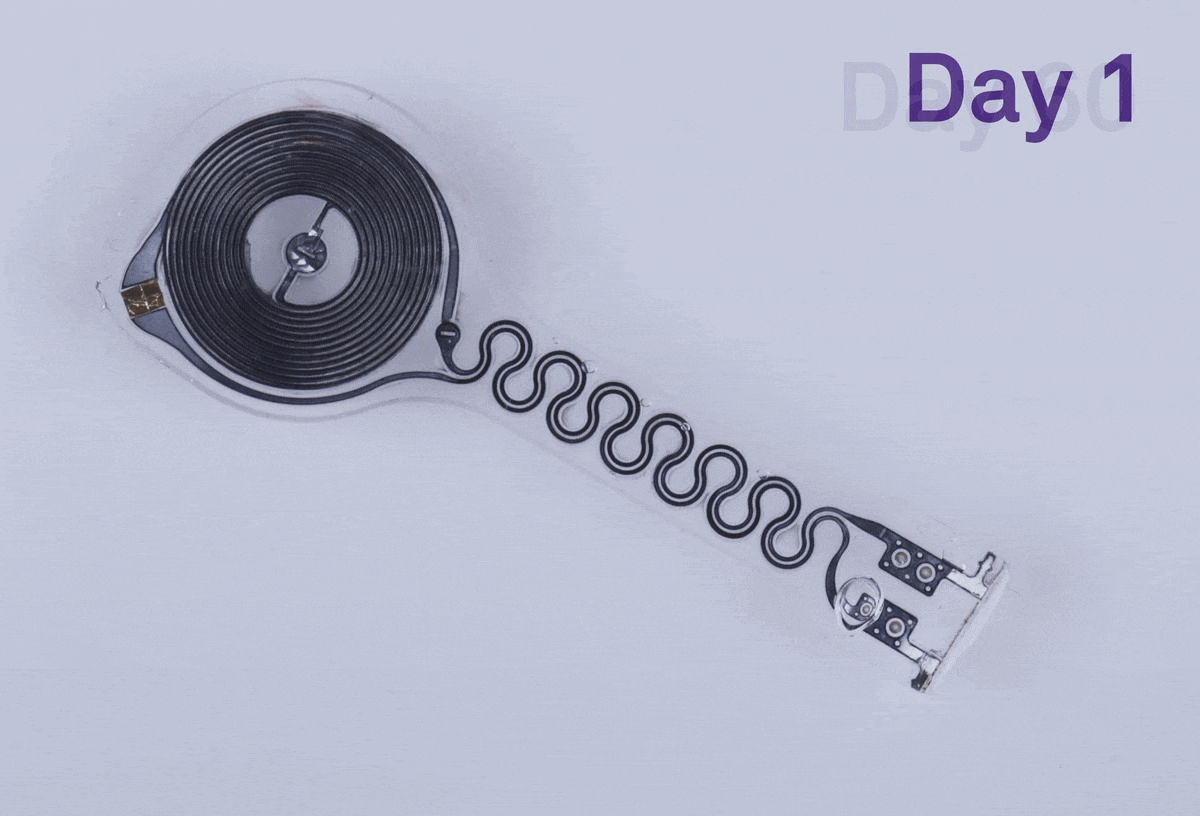
The thin, flexible device was designed to be fully biocompatible, meaning that none of its components would trigger a toxic or immune response from the body. It also wouldn’t require batteries or leads — wires connected to the heart that allow a pacemaker to regulate a person’s heart beat by sending electrical pulses — to operate. And it was made to be bioresorbable, with water-soluble metals and other components that dissolve in bodily fluid over several weeks. When a pacemaker needs to be removed or replaced, doctors have to surgically extract the leads and electrodes attached to the heart. While this procedure is typically safe, it can raise the risk of infection or other complications. Those are risks that the team’s device would in theory be able to avoid completely.
In their new study, published Thursday in Science, the group has added more features to their pacemaker. According to author Igor Efimov, a professor of biomedical engineering and professor of medicine at Northwestern University, the pacemaker now comes with a “fully integrated network of wearable devices” attached to a patient’s skin, four in total.
These devices not only monitor a person’s heartbeat and other vital signs like body temperature — they also wirelessly power the pacemaker and control its pacing automatically as needed. Doctors can remotely monitor the data collected by the device via a computer network. And in experiments with living rodents and dogs, as well as human hearts in the lab, the pacemaker and its closed loop system seemed to work as intended.

Efimov estimates that tens of thousands of patients could benefit from this technology. “These patients include newborns with heart defects, adults after surgery to repair a heart valve or bypass a blocked coronary artery, or other patients who need a temporary pacemaker before a permanent one can be installed,” he said.
These new findings, while important, still represent the early stages of research. Efimov and his team plan to conduct larger animal studies of their tech before they move on to human trials. But they have already received interest from several companies and venture firms interested in helping further develop it. And they believe that if everything continues to go well, their device could reach the clinic within the next two to three years.
Right now, their pacemaker is only intended to be implanted outside the heart, and it’s proven to be more of a challenge to create one that can safely dissolve inside the heart. But should they overcome that hurdle, it would allow the technology to become even more broadly useful, Efimov said.
