remdesivir
-
FDA Approves Remdesivir to Treat Hospitalized Covid-19 Patients Despite Mixed Trial Results
The Food and Drug Administration has approved remdesivir to treat hospitalized covid-19 patients. The antiviral drug, which is manufactured and sold by the pharmaceutical company Gilead under the brand name Veklury, is the first to be approved for treating the virus in the U.S.
-
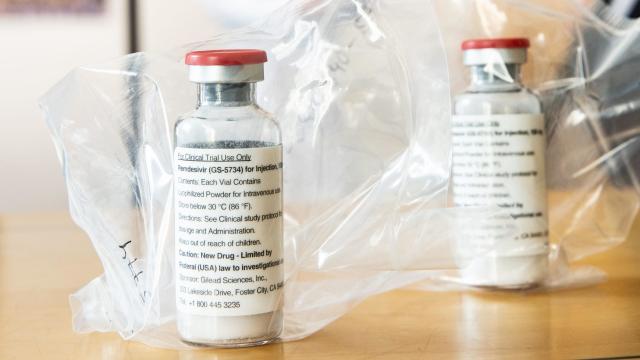
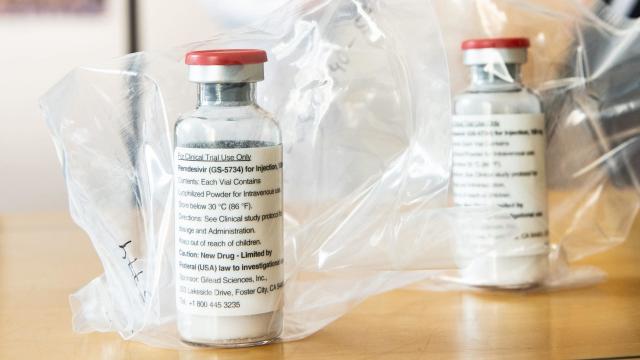
Gilead to Charge $4,550 Per Patient for Typical Covid-19 Treatment
The U.S. pharmaceutical company behind a drug that’s been approved by the Food and Drug Administration for emergency use for covid-19 patients announced today the drug will cost $US3,120 ($4,553) per typical treatment course for individuals with private insurance plans.
-
US To Allow Emergency Use Of Experimental Drug As Studies Show Mixed Results For Treating Covid-19
A wave of results from studies involving remdesivir, an experimental antiviral being used to treat covid-19, has arrived today. The data so far doesn’t provide a concrete answer as to how effective or life-saving the drug truly is for coronavirus patients. Nonetheless, the New York Times reports that the U.S.’s Food & Drug Administration is…